市场准入
Helping You Formulate a Market Access Strategy that Maximizes Access for Your Brand
With more expensive drugs winning regulatory approval and increased scrutiny on drug pricing, market access has never been more challenging and competitive. Our team offers a full spectrum of solutions and services to help you navigate the market access landscape and maximize access for your brand.
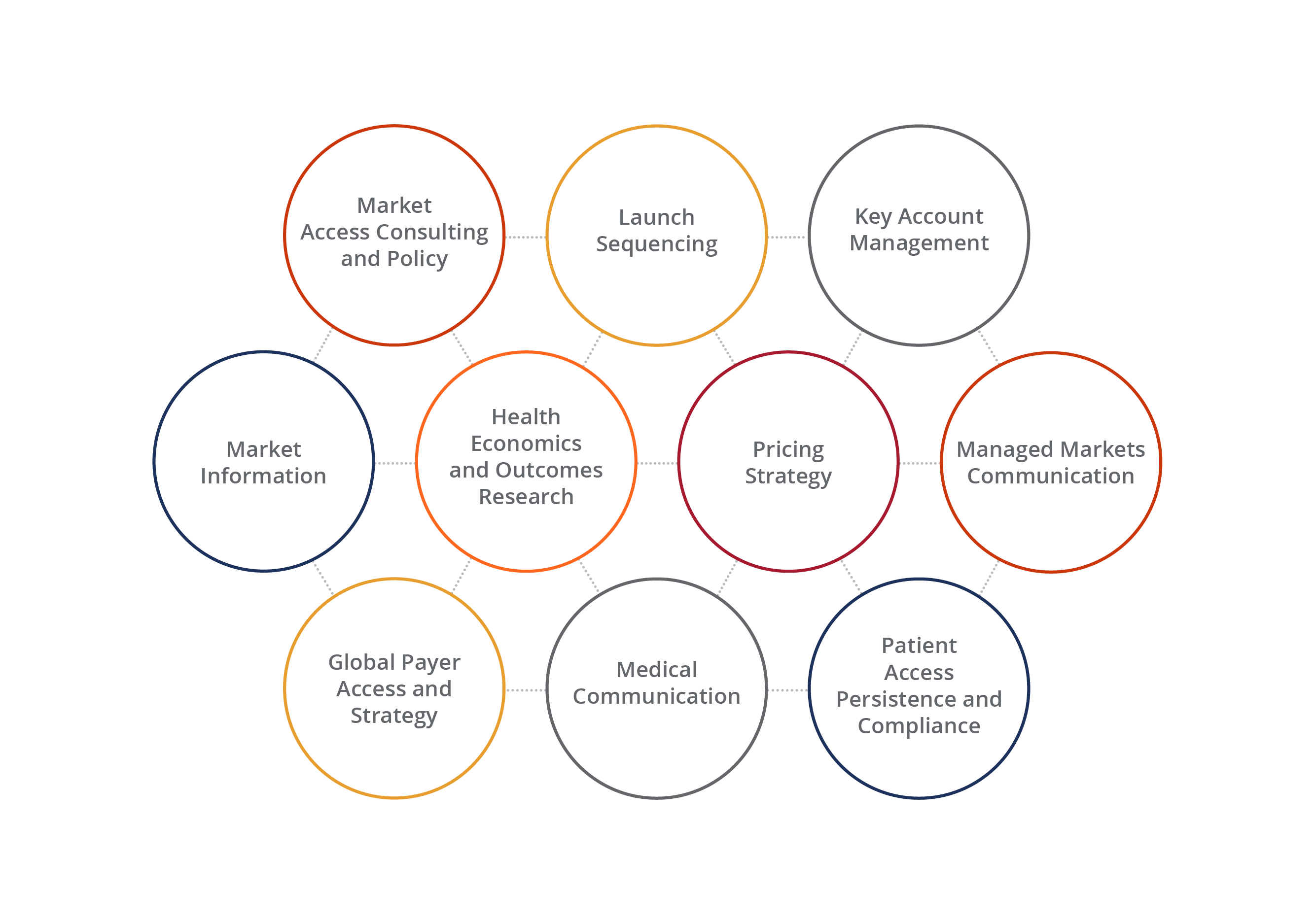
Optimizing Access to Markets Throughout Your Brand’s Life Cycle
Wherever you are in your brand’s life cycle, there is a team that can help you to achieve your access-related objectives. From research to strategy and tactical execution, we offer a comprehensive suite of services to give your brand and organization a competitive edge every step of the way.
A Complete Toolbox for Global Market Access
Whether it’s gathering market information or conducting cost analyses, we have the depth and resources to give our customers the insights they want and need. We bring these insights to life through our analytics, as well as our creative executions, generating captivating content that best communicates our customers’ strategy-driven messages.
Our Global Market Access Offering:
- Market assessment and analysis
- Comparative effectiveness research
- Pricing reimbursement and HEOR
- HCEI communications
- Patient assistance services
- Legislative and regulatory analysis
A Fit for All Shapes and Sizes
Whether you’re working for a company with a history of blockbuster launches or a start-up with a promising pipeline, we can help you develop and implement your market access strategy. Our multiple teams have collective experience across the healthcare continuum and can provide the support and services specific to your organization and brand, always tailoring them to help you overcome your brand-specific access challenges.
Close Connections on a Global Scale
With experienced teams in multiple countries, we understand the policies, trends and nuances in markets around the world.
BUILDING AND COMMUNICATING STRONG AND EFFECTIVE VALUE PROPOSITIONS
The complex, heterogeneous, and rapidly-changing payer environment is becoming increasingly difficult to navigate. Our expert teams of industry leaders, former payers, policy experts, and experienced strategists can help you navigate complex evidence requirements and anticipate and overcome payer barriers to help you achieve optimal price and access. Our team of communication specialists can help you develop compelling arguments presented in an accurate, succinct, and engaging story format to shape effective payer positioning and position evidence to overcome payer objections.
WE CAN HELP YOU
Gather in-depth information on the HTA, pricing, and reimbursement drivers and limiters, as well as landscape reviews, analog and market assessments
- Develop comprehensive global pricing and market access strategies and recommendations
- Develop evidence generation strategies to support optimal value positioning, HTA assessment, and pricing
- Support in-licensing decisions with rapid turnaround insights into global pricing and reimbursement potential
- Provide cross-functional alignment on pricing, access and evidence strategies through market access workshops
- Craft value stories that articulate a logical flow of arguments, supported by available evidence
- Test, refine, and validate value messages with relevant stakeholders
- Present payer-relevant evidence through global value dossiers, slide decks, objection handlers, and training
- Develop country-specific pricing and reimbursement or formulary submissions, including Academy of Managed Care Pharmacy (AMCP) dossiers for U.S. payers and HTA submissions for various markets
- Convey messages and evidence in a dynamic, user-friendly way electronically through iValue Suite
- Explore key market access hypotheses through international payer advisory boards
WE OFFER YOU
- A network of over 2,000 payer experts and advisors worldwide
- A Pricing and Reimbursement Policy Council (PRPC) comprised of a diverse group of industry experts, and providing insights into the latest issues, trends, and changes affecting market access
- Expertise and experience in over 45 countries and across all major therapeutic areas
- In-house expertise and no outsourcing, with ~35,000 payer interviews and all value story and dossier writing done internally
- Rigorous quality standards applied to all content development
ADDRESSING THE LATEST TRENDS IN MARKET ACCESS
- Advanced Therapy Medicinal Products
- Early Access
- Expanded Access
- Conditional Marketing (e.g,, for Orphan Drugs)
- Medical Devices
- Price Referencing
- Surrogate Endpoint Use
- Transparency in Drug Pricing
研究项目管理
Effective communication of evidence and information is essential to conveying the value of your products, both to internal and external stakeholders. With approximately 20 years of experience, our highly trained staff provide medical writing services for the peri- and post-approval phases of the product lifecycle, guaranteeing a consistency in quality and voice. Our writers prepare documents that are clear, concise, compelling, and scientifically accurate, while also ensuring they are fully compliant with regulations, industry best practices, and your corporate guidance.
- Site Identification
- KOLs networking
- PI Training
- Clinical Audit
- online SMO
上市后研究
专业团队为中国上市后研究助力
梅斯医学真实世界研究BU在上市后临床研究的设计和执行方面具有广泛的经验。我们的研究通过结构化的研究设计和执行产生有影响的医学数据,以及产品或特定患者群体的医学证据,以更好地指导临床实践,实现最市场准入和最优商业,并满足监管当局的要求。我们所有的研究都是按照GCP规范和相应研究指南进行的。
梅斯医学开发针对上市后研究的精简且经济有效的标准操作规程,最大限度地收集数据、监控和质量,同时最大限度地减少操作风险。
梅斯医学真实世界研究团队具有深厚的政策和专业知识,包括伦理和监管要求、患者隐私立法、调查人员补助政策、学术和网站网络、患者协会、国家卫生数据库和本地卫生保健系统
卓越的研究设计与执行
梅斯医学真实世界研究BU在上市后临床研究的设计和执行方面具有广泛的经验。我们的研究通过结构化的研究设计和执行产生有影响的医学数据,以及产品或特定患者群体的医学证据,以更好地指导临床实践,实现最市场准入和最优商业,并满足监管当局的要求。我们所有的研究都是按照GCP规范和相应研究指南进行的。
梅斯医学开发针对上市后研究的精简且经济有效的标准操作规程,最大限度地收集数据、监控和质量,同时最大限度地减少操作风险。
梅斯医学真实世界研究团队具有深厚的政策和专业知识,包括伦理和监管要求、患者隐私立法、调查人员补助政策、学术和网站网络、患者协会、国家卫生数据库和本地卫生保健系统
药品重点监测
梅斯医学真实世界研究BU在上市后临床研究的设计和执行方面具有广泛的经验。我们的研究通过结构化的研究设计和执行产生有影响的医学数据,以及产品或特定患者群体的医学证据,以更好地指导临床实践,实现最市场准入和最优商业,并满足监管当局的要求。我们所有的研究都是按照GCP规范和相应研究指南进行的。
梅斯医学开发针对上市后研究的精简且经济有效的标准操作规程,最大限度地收集数据、监控和质量,同时最大限度地减少操作风险。
梅斯医学真实世界研究团队具有深厚的政策和专业知识,包括伦理和监管要求、患者隐私立法、调查人员补助政策、学术和网站网络、患者协会、国家卫生数据库和本地卫生保健系统
真实世界研究
真实世界证据的增长需求
梅斯医学的药物安全和药物警戒团队支持药械的临床试验和上市后安全监查,为我们客户提供产品整个生命周期内独立和整体服务。
让我们与众不同的是我们的员工:高素质的药物安全科学家和熟练的医疗专业人员,他们在患者管理,特定的药物警戒服务以及监管法规方面,拥有深厚的专业知识和药物安全管理丰富的经验。

我们可以协助您:
-
描绘自然病史和病程(例如,发病率,患病率,护理标准)
-
通过描述疾病负担来确定未满足的临床和人文需求
-
收集罕见疾病人群数据
-
量化真实世界产品特定和/或比较安全性,有效性,依从性和其他结局
-
评估特定的治疗模式,量化相关的护理成本,并填充卫生经济模型
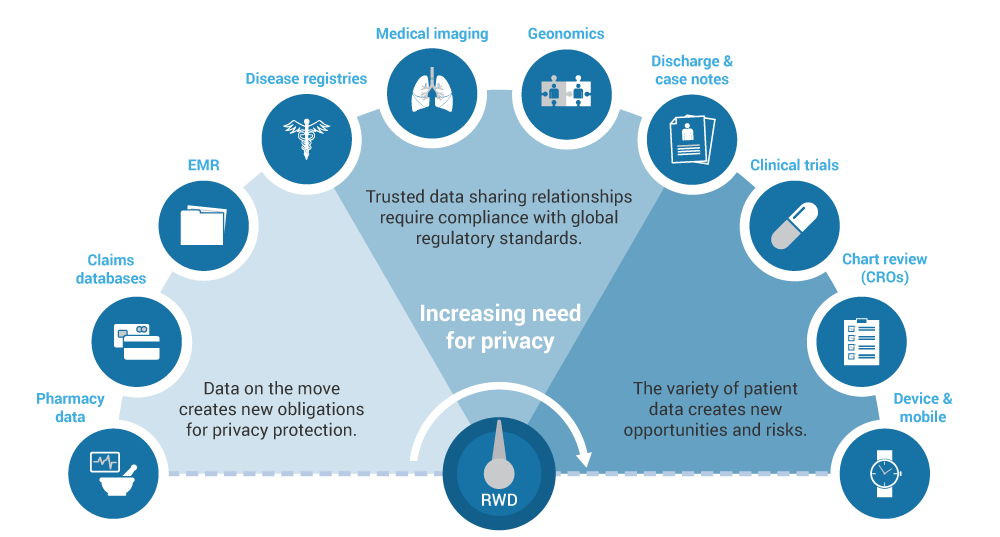
深入的数据洞察力
我们专注于您的研究问题,并提供包含各种实际数据源和数据的最佳解决方案。我们由健康经济学家,流行病学家,生物统计学家和临床医生组成的综合团队对可用的数据库进行严格评估,以确定那些能够提供最佳信息以满足您的研究需求的数据库。我们对来自20多个国家(包括北美和欧洲)以及其他地区(如巴西,日本,韩国,中国,澳大利亚,台湾)的数据来源有深入的了解和经验。我们了解并可以分析大型索赔数据库和电子病历以及这些和其他数据源的组合。
我们专注于您的研究问题,并提供包含各种实际数据源和数据的最佳解决方案。我们由健康经济学家,流行病学家,生物统计学家和临床医生组成的综合团队对可用的数据库进行严格评估,以确定那些能够提供最佳信息以满足您的研究需求的数据库。我们对来自20多个国家(包括北美和欧洲)以及其他地区(如巴西,日本,韩国,中国,澳大利亚,台湾)的数据来源有深入的了解和经验。我们了解并可以分析大型索赔数据库和电子病历以及这些和其他数据源的组合。
与专业机构、协会、学会、Kols的深入合作
实现最佳市场准入和有效商业化需要定制化的研究设计,深入的专业知识以及对全国Kols的关系网络。我们对上市后环境的理解加上十数年临床研究的经验,转化为独有的能力,可提供量身定制的有效研究设计,以满足特定目标和市场需求和产品的监管要求。此外,我们的运营模式是根据医疗,临床,项目管理,监管,医保和流行病学职能部门的专家意见量身定制的,以满足项目特定目标和利益相关者的期望。
卓越的研究设计和执行
- 丰富的经验 - 专注于真实世界研究设计、运营、监察和成果团队,15个重点临床研究领域,200多个合作研究点,500多位长期合作研究者,7万多主任医师会员,200万临床医师资源
- 专业的研究团队 - 在早期参与合作模式下工作时,在研究启动和患者招募时间方面大大优于行业基准
- 治疗领域专家 - 具有广泛适应症的能力,以及利用我们的临床医生和全国运营专业人员网络的能力
- 通过技术和合作创新 - 充分利用人工智能和大数据技术,以及真实世界研究的特点,大幅降低研究成本,提高操作的效率,确保高质量的研究成果
真实世界数据
Effective communication of evidence and information is essential to conveying the value of your products, both to internal and external stakeholders. With approximately 20 years of experience, our highly trained staff provide medical writing services for the peri- and post-approval phases of the product lifecycle, guaranteeing a consistency in quality and voice. Our writers prepare documents that are clear, concise, compelling, and scientifically accurate, while also ensuring they are fully compliant with regulations, industry best practices, and your corporate guidance.
- Real-world data collection
- patient or physician surveys
- Hybrid database studies
- Product Registries
- Disease Registries
- Electronic clinical outcome assessment eCOA (ePRO/eClniRO/eObsRO)











